Medtronic Insulin Pump Therapy
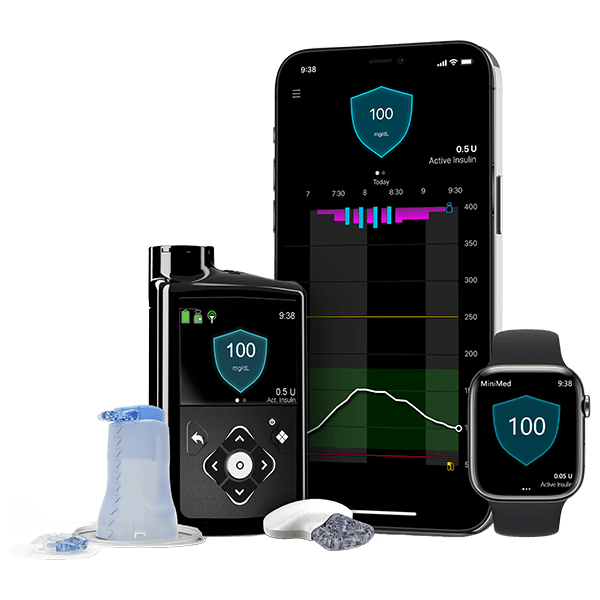
MiniMed™ 780G Insulin Pump System
Designed for real life.
Ever miss a meal dose sometimes or underestimate a carb count? Life with diabetes can be unpredictable. Now there’s an insulin pump system designed with meal detection technology*** that automatically adjusts and corrects† sugar levels every 5 minutes§.
For those times when you forget to plan ahead. Just like in real life.
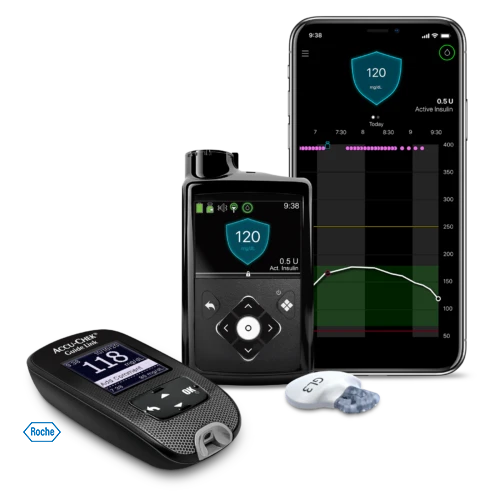
MiniMed™ 770G Insulin Pump System
The MiniMed™ 770G insulin pump system automatically adjusts background insulin every 5 minutes.* Using real-time glucose readings, the system is able to calculate a personalized amount of insulin to deliver based on CGM. The system connects directly with a compatible smartphone, allowing you to view sugar trends and insulin delivery on the go.
 Pump automatically adjusts background insulin every 5 minutes to help you stay in target range.*
Pump automatically adjusts background insulin every 5 minutes to help you stay in target range.*
 See insulin delivery, sugar trends, and high and low alerts on your smartphone by downloading the app.
See insulin delivery, sugar trends, and high and low alerts on your smartphone by downloading the app.
 The MiniMed™ 770G system is enabled for future software upgrades, when available.**
The MiniMed™ 770G system is enabled for future software upgrades, when available.**
 Your insulin pump data can be shared with your healthcare provider, family, and care team for peace of mind.*
Your insulin pump data can be shared with your healthcare provider, family, and care team for peace of mind.*

MiniMed™ 630G Insulin Pump System
With optional CGM, the MiniMed™ 630G insulin pump connects with the Guardian™ Sensor 3 to continuously check your glucose levels detecting highs and lows. It works with SmartGuard™ technology to help you stay within your target range.
With the pump and sensor system, you’re four times more likely to reach your target A1C1. You can also reduce low glucose episodes by up to 84 percent and lower the risk of long-term complications2.
FREQUENTLY ASKED QUESTIONS
The MiniMed™ 770G System is indicated for adults or children (ages 2 and older) with Type 1 diabetes. The MiniMed™ 630G System is indicated for people with diabetes mellitus (ages 14 and older for GS3 sensor; ages 16 and older for Enlite sensor).
Yes, there are two smartphone apps for MiniMed™ 770G insulin pump users. The MiniMed™ Mobile app displays your diabetes data allowing you to easily track your sugar levels and get notified if you are going high or low. The Carelink™ Connect app is available for care partners to keep everyone in the loop. Up to five people can follow a pump user and receive high and low notifications.
For qualified beneficiaries, Medicare covers insulin pump therapy and continuous glucose monitoring that integrates with a Medtronic insulin pump. Once you’ve completed our online application, we will work with you, your healthcare provider, and insurance company to help you get the insulin pump and supplies you need. If you have any questions, please reach out to our Diabetes Care team at DCA@myehcs.com.
Fingersticks are required to calibrate the system twice a day. A fingerstick is also required if therapy adjustments are needed.
Once you complete the Medtronic application, EHCS will pair you with a dedicated Diabetes Care Advisor to help you through the process. From insurance approval to future reorders, our team of DCAs is your guide to better diabetes control.
Most insulin pumps have a standard 4-year replacement warranty. If you’re interested in upgrading your insulin pump, please reach out to our team of Diabetes Care Advisors at DCA@myehcs.com. They will know your pump’s warranty time, upgrade timeframe, and criteria for upgrading.

EHCS is an authorized distributor of Medtronic insulin pump therapy. We strive to provide you with quality products and multiple options.
* Refers to SmartGuard™ Auto Mode. Some user interaction required. Individual results may vary.
** The MiniMed™ 770G system is enabled for future software upgrades; when such software upgrades are available, customers will be offered a software upgrade. Program eligibility requirements and terms conditions apply. Subject to change.
*** Taking a bolus 15-20 minutes before a meal helps to keep blood sugar levels under control after eating.
1 Aronson R, et al. Diabetes Obes Metab. 2016. e-pub ahead of print. DOI: 10.1111/dom.12642
2 DCCT Research Group. N Engl J Med. 1993;329:977-986
† Refers to auto correct, which provides bolus assistance. Can deliver all auto correction doses automatically without user interaction, feature can be turned on and off.
§ Refers to SmartGuard™ feature. Individual results may vary.
Important safety information: MiniMed™ 780G system with SmartGuard™ technology with Guardian™ 4 sensor
The MiniMed™ 780G system is intended for continuous delivery of basal insulin at selectable rates, and the administration of insulin boluses at selectable amounts for the management of type 1 diabetes mellitus in persons seven years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 780G system includes SmartGuard™ technology, which can be programmed to automatically adjust insulin delivery based on the continuous glucose monitoring (CGM) sensor glucose values and can suspend delivery of insulin when the sensor glucose (SG) value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 780G system consists of the following devices: MiniMed™ 780G insulin pump, the Guardian™ 4 transmitter, the Guardian™ 4 sensor, One-press serter, the Accu-Chek™ Guide Link blood glucose meter, and the Accu-Chek™ Guide test strips. The system requires a prescription from a healthcare professional.
The Guardian™ 4 sensor is intended for use with the MiniMed™ 780G system and the Guardian 4 transmitter to monitor glucose levels for the management of diabetes. The sensor is intended for single use and requires a prescription. The Guardian™ 4 sensor is indicated for up to seven days of continuous use.
The Guardian™ 4 sensor is not intended to be used directly to make therapy adjustments while the MiniMed™ 780G is operating in manual mode. All therapy adjustments in manual mode should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ 4 sensor. The Guardian™ 4 sensor has been studied and is approved for use in patients ages 7 years and older and in the arm insertion site only. Do not use the Guardian™ 4 sensor in the abdomen or other body sites including the buttocks, due to unknown or different performance that could result in hypoglycemia or hyperglycemia.
WARNING: Do not use the SmartGuard™ feature for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in the SmartGuard™ feature.
WARNING: Do not use the MiniMed™ 780G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 780G system.
WARNING: Do not use SG values to make treatment decisions, including delivering a bolus, while the pump is in Manual Mode. When the SmartGuard™ feature is active and you are no longer in Manual Mode, the pump uses an SG value, when available, to calculate a bolus amount. However, if your symptoms do not match the SG value, use a BG meter to confirm the SG value. Failure to confirm glucose levels when your symptoms do not match the SG value can result in the infusion of too much or too little insulin, which may cause hypoglycemia or hyperglycemia.
Pump therapy is not recommended for people whose vision or hearing does not allow for the recognition of pump signals, alerts, or alarms. The safety of the MiniMed™ 780G system has not been studied in pregnant women, persons with type 2 diabetes, or in persons using other anti-hyperglycemic therapies that do not include insulin. For complete details of the system, including product and important safety information such as indications, contraindications, warnings and precautions associated with system and its components, please consult https://www.medtronicdiabetes.com/important-safety-information#minimed-780g and the appropriate user guide at https://www.medtronicdiabetes.com/download-library
Important Safety Information: MiniMed™ 770G System With SmartGuard™ Technology
The MiniMed™ 770G system is intended for continuous delivery of basal insulin (at user selectable rates) and administration of insulin boluses (in user selectable amounts) for the management of type 1 diabetes mellitus in persons two years of age and older requiring insulin as well as for the continuous monitoring and trending of glucose levels in the fluid under the skin. The MiniMed™ 770G System includes SmartGuard™ technology, which can be programmed to automatically adjust delivery of basal insulin based on continuous glucose monitoring (CGM) sensor glucose values (SG) and can suspend delivery of insulin when the SG value falls below or is predicted to fall below predefined threshold values.
The Medtronic MiniMed™ 770G System consists of the following devices: MiniMed™ 770G Insulin Pump, the Guardian™ Link (3) Transmitter, the Guardian™ Sensor (3), one-press serter, the Accu-Chek® Guide Link blood glucose meter, and the Accu-Chek® Guide Test Strips. The system requires a prescription.
The Guardian™ Sensor (3) has not been evaluated and is not intended to be used directly for making therapy adjustments, but rather to provide an indication of when a fingerstick may be required. All therapy adjustments should be based on measurements obtained using a blood glucose meter and not on values provided by the Guardian™ Sensor (3).
All therapy adjustments should be based on measurements obtained using the Accu-Chek® Guide Link blood glucose meter and not on values provided by the Guardian™ Sensor (3). Always check the pump display to ensure the glucose result shown agrees with the glucose results shown on the Accu-Chek® Guide Link blood glucose meter. Do not calibrate your CGM device or calculate a bolus using a blood glucose meter result taken from an alternative site. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise.
WARNING: Do not use the SmartGuard™ Auto Mode for people who require less than 8 units or more than 250 units of total daily insulin per day. A total daily dose of at least 8 units, but no more than 250 units, is required to operate in SmartGuard™ Auto Mode.
WARNING: Do not use the MiniMed™ 770G system until appropriate training has been received from a healthcare professional. Training is essential to ensure the safe use of the MiniMed™ 770G system.
Important Safety Information: MiniMed™ 630G System with SmartGuard™ Technology
Indicated for the continuous delivery of insulin, at set and variable rates, for the management of diabetes mellitus. MiniMed™ 630G system is approved for ages 14 years or older with Guardian™ Sensor 3 and MiniMed™ 630G system is approved for ages 16 years or older with Enlite™ sensor. Both systems require a prescription. Insulin infusion pumps and associated components of insulin infusion systems are limited to sale by or on the order of a physician and should only be used under the direction of a healthcare professional familiar with the risks of insulin pump therapy. Pump therapy is not recommended for people who are unwilling or unable to perform a minimum of four blood glucose tests per day. Pump therapy is not recommended for people who are unwilling or unable to maintain contact with their healthcare professional. Pump therapy is not recommended for people whose vision or hearing does not allow recognition of pump signals and alarms. Insulin pumps use rapid-acting insulin. If your insulin delivery is interrupted for any reason, you must be prepared to replace the missed insulin immediately. Replace the infusion set every 48–72 hours, or more frequently per your healthcare professional’s instructions. Insertion of a glucose sensor may cause bleeding or irritation at the insertion site. Consult a physician immediately if you experience significant pain or if you suspect that the site is infected. The information provided by CGM systems is intended to supplement, not replace, blood glucose information obtained using a blood glucose meter. A confirmatory fingerstick using a CONTOUR®NEXT LINK 2.4 meter is required prior to making adjustments to diabetes therapy. Always check the pump display when using a CONTOUR®NEXT LINK 2.4 meter, to ensure the glucose result shown agrees with the glucose results shown on the meter. Do not calibrate your CGM device or calculate a bolus using a result taken from an Alternative Site (palm) or a result from a control solution test. If a control solution test is out of range, please note that the result may be transmitted to your pump when in the “Always” send mode. It is not recommended to calibrate your CGM device when sensor or blood glucose values are changing rapidly, e.g., following a meal or physical exercise. The MiniMed™ 630G system is not intended to be used directly for preventing or treating hypoglycemia but to suspend insulin delivery when the user is unable to respond to the Suspend on low alarm and take measures to prevent or treat hypoglycemia themselves. Therapy to prevent or treat hypoglycemia should be administered according to the recommendations of the user’s healthcare provider.
WARNING: The SmartGuard™ Suspend on low feature will cause the pump to temporarily suspend insulin delivery for two hours when the sensor glucose reaches a set threshold. Under some conditions of use the pump can suspend again, resulting in very limited insulin delivery. Prolonged suspension can increase the risk of serious hyperglycemia, ketosis, and ketoacidosis. Before using the SmartGuard™ feature, it is important to read the SmartGuard™ feature information in the User Guide and discuss proper use of the feature with your healthcare provider.

